- Do you have an innovative and transformative idea?
- Results and capabilities portfolio
- Collaborative innovation
- Funded Innovation Projects
- Up to date in innovation
- Biopolo La Fe - Business collaboration space
Extracellular vesicles with improved therapeutic capacity

- Area:
- Cardiovascular Pathology
- Group:
- Regenerative Medicine and Heart Transplantation
- Type:
- Patent
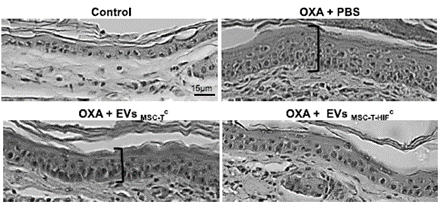
Chronic inflammation is a pathophysiological state caused by the uncontrolled activation of the immune system A wide variety of diseases cause chronic inflammation, such as graft-versus-host disease, acute rejection of a transplanted organ or autoimmune pathologies.
Mesenchymal stromal cells and the released extracellular vesicles (EVs) have demonstrated immunomodulatory capacity in preclinical setting, but when this therapies were transferred to clinical arena the results were inconclusive. In this technology, the IIS La Fe team has genetically modified wild type MSC and generated extracellular vesicles (EVs) with greater therapeutic potency than those releases by wild type MSCs.
The invention presents the generation of a new line of stromal mesenchymal cells that overexpress HIF-1α and hTERT through genetic modification, which is cultured in the presence of secreted TNF-α, IFN-γ and IL-1β for 48 hours (EVs) with a greater therapeutic potential than those secreted by unenhanced mesenchymal stem cells (MSCs).
These new EVs exert a greater therapeutic effect and in turn allow unlimited cell growth to obtain a stable and homogeneous source of vesicles with improved therapeutic functions. By overexpression of HIF-1α, therapeutic improvement is obtained, while overexpression of hTERT generates unlimited and constant cell division. The main advantage of the invention is the combination in the use of genetic modifications with the specific culture protocol.
Business application sectors
The main sector interested in this invention is composed of the pharmaceutical companies that are currently working on the development of therapies based on stromal mesenchymal cells or extracellular vesicles.
Technical advantages and business benefits
- Provides a greater immunosuppressive capacity to the vesicles secreted by pulp-derived stromal mesenchymal cells.
- Stromal mesenchymal cells do not enter replicative senescence donors every little time.
- Induces secretion of EVs with greater immunoregulatory capacity.
State of technology development
The results have been validated in murine models of ischemia, ulcerative colitis and delayed hypersensitivity with satisfactory results. Work is being done on scaling up the production of these EVs under GLP / GMP conditions and on regulatory documentation to be able to use this biological product in a First in man clinical trial.
Intellectual Property Rights
National Patent EP20383170, dated December 29, 2020.
Title: Extracellular vesicles derived from mesenchymal stromal cells genetically modified to overexpress HIF-1A and hTERT.
Collaboration wanted
The inventors are looking for investors and companies interested in licensing the technology, as well as strategic partners and new sources of funding to advance development.

