News
Researchers at IIS La Fe and the UV advance in the integration of metabolomics in the in vitro study of hepatotoxicity by new drugs.
This is the conclusion of a study that places metabolomics as a disruptive technology to improve the study of the potential hepatotoxicity of new drugs.
Metabolomics could identify the potential hepatic toxicity of a drug in the early stages of development and thus help to select more effective and less toxic options.

An article by the Joint Research Unit in Experimental Hepatology of the La Fe Health Research Institute (IIS La Fe) has demonstrated the potential of metabolomics for the study of hepatotoxicity of new drugs thanks to its relevance as a tool for discovering new biomarkers.
Hepatotoxicity or Drug-Induced Liver Disease (DILI) is one of the most frequent adverse clinical reactions and a relevant cause of morbidity and mortality. It is a major cause of drug withdrawal, both in the early stages of development and when approved and in clinical use. It is therefore a crucial concern for the pharmaceutical industry, with health and economic repercussions.
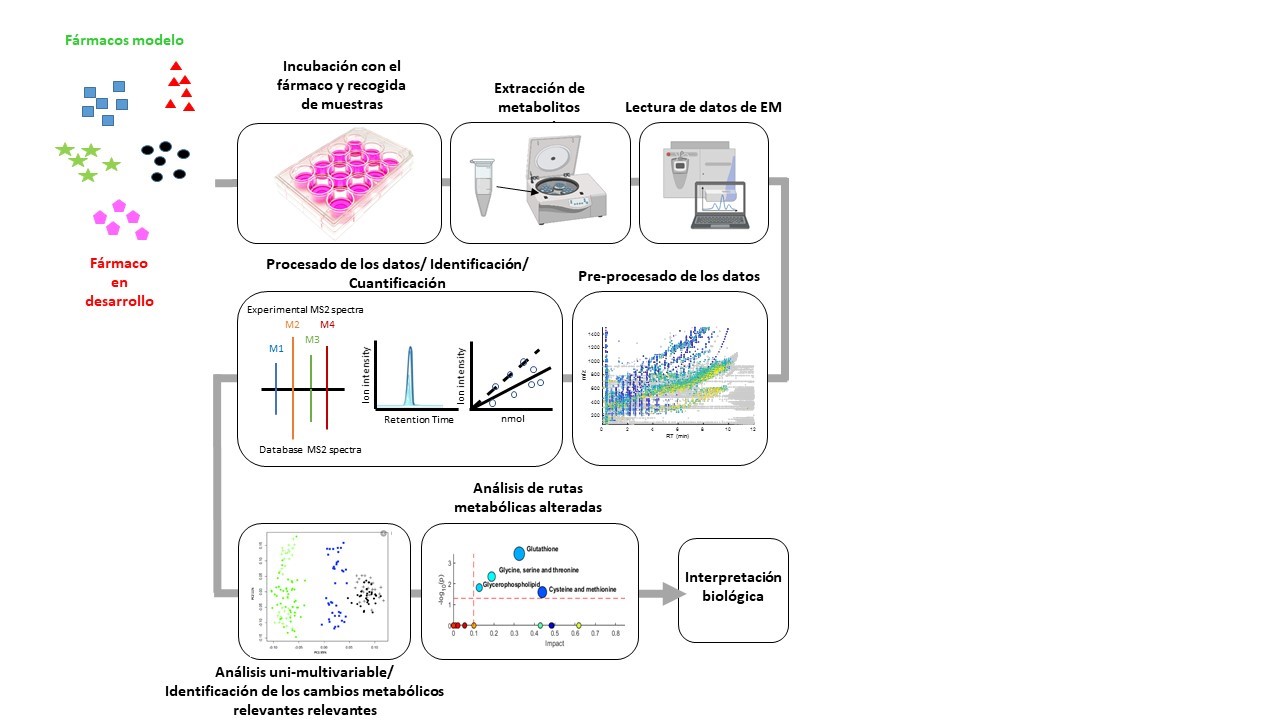
The team of researchers from IIS La Fe, Universitat de València (UV), integrated in the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), has studied various aspects of drug hepatotoxicity, in order to identify new biomarkers for DILI based on metabolomics. This work has confirmed that the integration of metabolomics for the study of hepatotoxicity in both in vivo studies (with patients who have experienced episodes of hepatotoxicity) and in vitro (using cultured hepatocytes as a model of the human liver) provides a valuable information to further investigate the mechanisms of hepatotoxicity and to anticipate its effects.
Identifying and modifying hepatotoxicity
In this way, an innovative, highly sensitive tool is integrated, allowing to successfully address the investigation of the potential hepatotoxicity of new drugs at very early stages of their development, thus avoiding a premature withdrawal from the market due to the late detection of severe hepatotoxic events.
"Achieving an ultra-sensitive method to detect metabolic changes that is at the same time robust, reproducible and provides comparable results, even when experiments are performed at different times, has been a challenging task. This aspect has been fundamental to the success of this technology in the study of hepatotoxicity," explained Dr Marta Torres, a Ramón y Cajal researcher at UV, a member of the Joint Unit and author of the study.
"The main challenge remains the ability to identify the possible hepatotoxicity of a new drug in the early stages of development, with the aim of modifying it or selecting analogues with similar therapeutic effects and less toxic effects," said Dr. José V. Castell, emeritus researcher at IIS La Fe and head of the Joint Research Unit in Experimental Hepatology.
Consolidated track record in the study of drug hepatotoxicity and metabolomics
In the field of omics, disciplines that employ innovative and disruptive technologies to investigate biological phenomena, metabolomics has recently emerged as an outstanding tool in the field of biomedical research. Its advances have been remarkable, contributing significantly both to the development of biologically active compounds and to biomedical diagnostics.
The Joint Research Unit in Experimental Hepatology, belonging to the La Fe Health Research Institute (UV-IIS La Fe) and affiliated to the Centre for Biomedical Research Network on Liver and Digestive Diseases (CIBERehd) and the Centre for Bioengineering, Biomaterials and Nanomedicine (CIBER-Bbn), has a solid and proven track record in the study of drug hepatotoxicity. Over the last few years, he has been developing innovative strategies to examine in greater detail and precision the adverse effects that a drug could have on the liver.
The principle of toxicometabolomics, which refers to the application of metabolomics to the study of toxicity, is based on a fundamentally simple concept. Within each living cell, there are a variety of small molecules, known as metabolites, that are involved in the biochemical processes that take place within it. When a cell is exposed to a bioactive substance, that substance can interfere with normal cellular metabolism, altering the normal flow of these processes and thereby affecting the concentrations of these intracellular metabolites. Some of these alterations are irrelevant or harmless to the cell, while others involve significant metabolic changes, leading to dysfunction, toxicity and sometimes cell death. Therefore, by analysing the global changes that a new drug induces in the set of metabolites present in the cell (metabolome) and assessing their biochemical relevance, we can obtain a more accurate picture of its predicted hepatotoxic effects.
Until a few years ago, the analysis of the cell metabolome was genuinely complex. However, the introduction of a highly efficient separation method, such as high-performance liquid chromatography (HPLC), together with the precise detection of the mass of metabolites and the resulting fragments after electron-beam mass spectrometry, has greatly facilitated the precise identification of the metabolites present in the sample. In addition, the intensity of the signal provides information on changes in the relative abundance of these metabolites.
Quintás G, Castell JV and Moreno-Torres M (2023) The assessment of the potential hepatotoxicity of new drugs by in vitro metabolomics. Front. Pharmacol. 14:1155271. doi: 10.3389/fphar.2023.1155271







