- Do you have an innovative and transformative idea?
- Results and capabilities portfolio
- Collaborative innovation
- Funded Innovation Projects
- Up to date in innovation
- Biopolo La Fe - Business collaboration space
Endoscopic Smart Center

- Area:
- Digestive and Hepatic Pathology
- Group:
- Perioperative Medicine, Anesthesiology and Revival
- Type:
- Patent
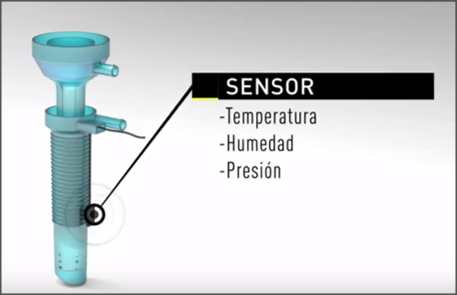
Endoscopic Smart Center consists of a device based on the integration of temperature, pressure and humidity sensors in the trocar needed for endoscopic surgery.
In order to perform endoscopic surgery, such as laparoscopic surgery, the creation of an operating space is needed by gas insufflation, usually CO2. Insufflation of CO2 provokes peritoneal damage due to low temperature and dryness. Monitoring of temperature, pressure and humidity is useful in order to variate these parameters and avoid tissue damage. However, existing devices in the market are unable to include all measures in one and varying parameters during operations can turn into a complicated performance.
The device designed by our team of surgeons and anesthetists consists of a modular system to control and monitor homeostasis during endoscopic surgeries. The modular system is made up of the device that is introduced in the internal cavity and a sensor and monitoring module for homeostasis control.
Business application sectors
The market application sector is either public or private healthcare systems. Public or private hospitals and health centers may acquire the device if they are performing endoscopic surgeries.
Technical advantages and business benefits
- It has a temperature and humidity sensors
- Possibility of modifying the insufflated gas parameters
- Individualization of applied pressure with volume control
- Image analysis with artificial intelligence
State of technology development
The development is in level TRL 6. We have obtained positive results in the tests performed in animals. The next steps include the final version of the prototype, clinical validation and regulatory compliance aspects for CE Marking.
Intellectual Property Rights
The device, co-ownership of IIS La Fe and Research Foundation of the General Hospital of Valencia, is registered in EPO by a European Patent EP19382791, with application date September 12th, 2019. Currently it is in international PCT extension.
Collaboration wanted
We seek the following collaborators:
-Investors to take the device to the final market phase: clinical validation and CE marking.
-Licensing to medical device company for commercialization of the device.

